Transcriptomics
-
Competing Endogenous RNA Networks in Colorectal Cancer
Stadler Group, Leipzig University, Service Center: RNA Bioinformatics Center - RBC
Colorectal cancer (CRC) is a heterogeneous cancer and its treatment can be different depending on its anatomical location. Some molecules can act as regulatory network influencing different aspects of cancer development. This study aimes to create competing endogenous RNA (ceRNA) networks to find biomarkers that elucidate clinical and functional implications for colon, rectum and rectosigmoid junction cancer via means of machine learning, based on RNA expression and clinical data of patients obtained from The Cancer Genome Atlas (TCGA).
For more information see RNA Bioinformatics Center (RBC)
Funded by: CAPES (Brazil)
Search projects by keywords:
-
Continuous Integration of RNAz, a bioinformatical research software for de novo detection of stable, conserved and functional noncoding RNAs in comparative genomics data
Stadler Group, Leipzig University, Service Center: RNA Bioinformatics Center - RBC
RNAz is a well established software to identify conserved RNA structures in genome-wide multiple sequence alignments. It has been widely used for more than a decade. RNAz makes use of the ViennaRNA package to compute secondary structures and their energies. Progress in the understanding of RNA folding, sequencing technologies, and alignment algorithms makes it necessary to restructure the RNAz software. In order to adapt RNAz to the expanded variations of energy models and to achieve longterm maintainability of this software we will devise automatized pipelines for the re-training of regression and decision models that are an essential part of RNAz and provide a repository of such models and corresponding datasets for the community.
For more information see RNA Bioinformatics Center (RBC)
Funded by: Federal Ministry of Education and Research 031A538B
Search projects by keywords:
-
LAMarCK - LongitudinAI Multiomics Characterization of disease using prior Knowledge
Zeller Group, Saez-Rodriguez Group, Schlesner Group, Bork Group, and Korbel Group, Service center: Heidelberg Center for Human Bioinformatics – HD-HuB
The need for proper integration of molecular omics data from human individuals is arguably one of the biggest challenges in current biomedical research, as these data cover different functional aspects that facilitate basic research, but also reveal differences between human physiological states with medical relevance (e.g. disease versus healthy, different disease subtypes etc.). To tackle this challenge, we formed a team of researchers with complementary areas of expertise from different Heidelberg institutions, all associated with the de.NBI node HD-HuB, which delivers services on human genomics and human microbiomics. Both these fields rely on similar kinds of data, which are increasingly coming from longitudinal studies assessing multiple omics approaches. Thus, they face similar technical and methodological challenges - and closer communication, as well as integrative approaches bridging both fields hold great promise for improved diagnostic, prognostic and therapeutic approaches, as is already becoming apparent for type-2-diabetes onset.
The LAMarCK consortium on the one hand aims to devise generic approaches to integrate heterogeneous longitudinal multi-omics data across microbiome and host, on the other hand it will also develop frameworks that make use of domain-specific prior knowledge on the underlying biological mechanisms for improved interpretability of the results. Funded by the BMBF (grant no. 031L0181A), the consortium is applying and benchmarking state-of-the-art machine learning approaches for longitudinal multi-omics data from microbial and host cells and augmenting these by integration with biomolecular knowledge bases. These new approaches will help dissect the molecular mechanisms underlying human diseases, such as colorectal cancer.
Search projects by Keywords:
-
Long non-coding RNAs in animals and plants
Stadler Group, Leipzig University, Service Center: RNA Bioinformatics Center - RBC
Over the last decades, it has become evident that non-coding RNAs (ncRNAs) are essential factors regulating the spatio-temporal gene expression in animals and plants. Small-ncRNAs like miRNAs have been in the focus of research for several years now revealing certain differences and commodities between the animal and plant small RNA world. However, so far no comprehensive study comparing these two kingdoms of life in regard to long-ncRNAs (lncRNAs) is available. This project aims at filling this knowledge gap, performing a comprehensive study of long non-coding RNAs (lncRNAs) in animals and plants, to identify features that can be used for the annotation of known and novel lncRNAs and their functions in these domains.
For more information see RNA Bioinformatics Center (RBC)
Funded by: Federal Ministry of Education (031A538B) and German Academic Exchange Service (57390771)
-
Machine Learning in translational (single-cell) omics
Group for Clinical bioinformatics & Machine learning in translational single-cell biology, University Hospital Tübingen, University Tübingen, Service Center: Center for Integrative Bioinformatics - CiBi
Cell subpopulations play a pivotal role in the initiation and progression of immune processes and complex diseases. We plan to perform CPU/GPU based computations to elucidate intra- and intercellular mechanisms of such cell subsets conferring their function and association to organism-level phenotypes. Specifically, computations will be performed for development to leverage single-cell measurement based studies to this end. Specifically, we developed machine learning approaches to identify rare, phenotype associated cell populations either via deep or shallow unsupervised or supervised learning. High-dimensional single-cell snapshot measurements are now increasingly utilized to study dynamic processes, complementing our recent efforts based on time-lapse measurements. However, it is not trivial to derive interpretable dynamic models from these. To this end we developed and will further benchmark sparse regression approaches, probabilistic graphical modeling, supervised surrogate learning approaches for large scale reaction network inference and the Dynamic Distribution Decomposition, as well as psupertime, a supervised pseudotime approach for single-cell RNA seq time series based on a regression model, which explicitly uses time series labels as input. Methods will be benchmarked on both unsensitive research data, as well as applied on possibly sensitive patient data from collaborations with partners at the University/University Hospital Tübingen. Initial activity in this project involved simulation and AI based inference of T cell exhaustion differentiation in chronic infections (see Figure).
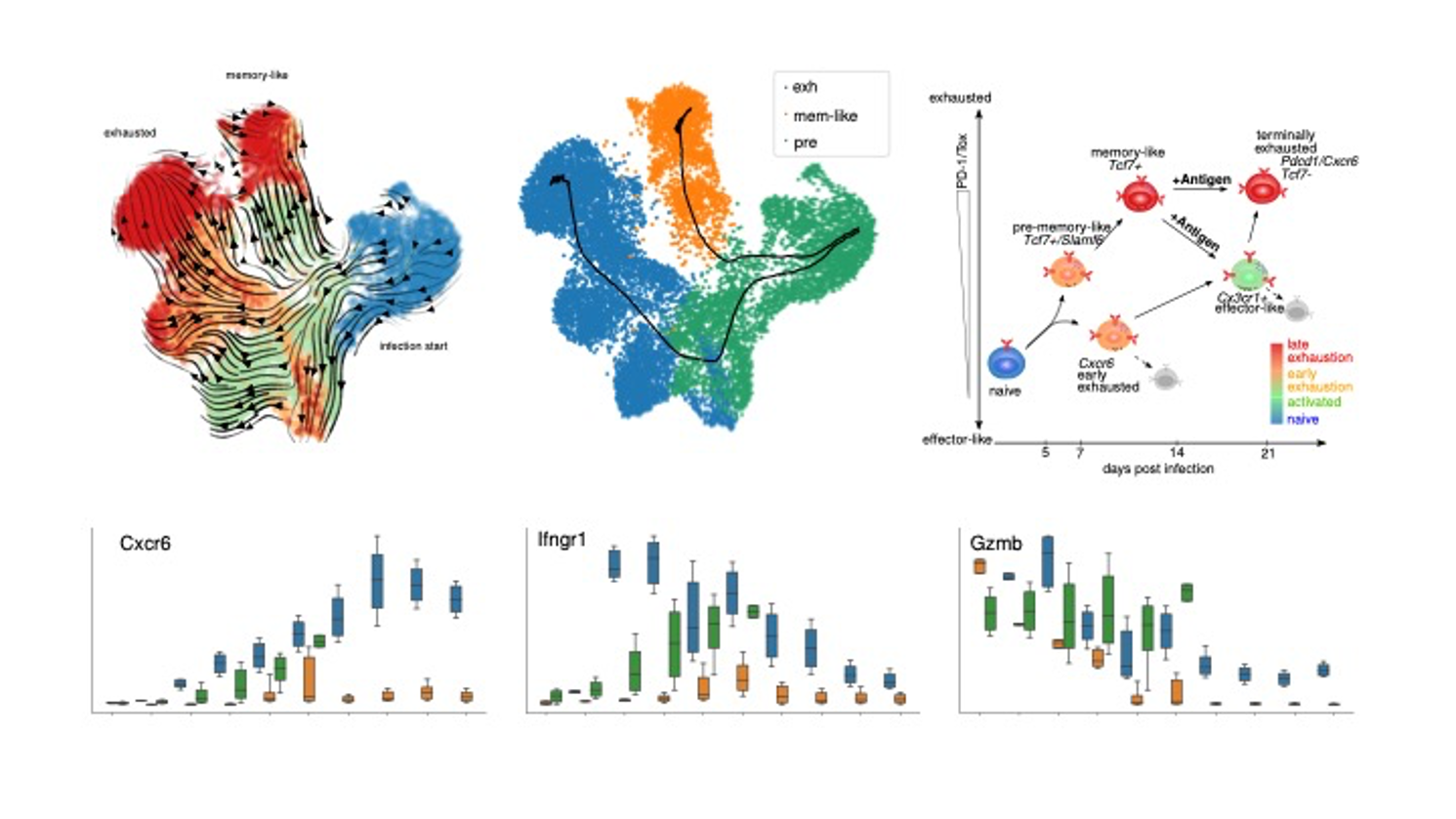
Simulation and AI based inference of T cell exhaustion differentiation in chronic infections. Dynamic and Markov Chain modeling of cellular bifurcated differentiation processes including classification based molecular characterization of individual differentiation branches. These analyses and follow up experiments (data not shown) resulted in high level differentiation model (Cerletti et al., in prep.).
Search projects by keywords:
-
Machine Learning-based classification of lncRNAs as ncRNA host genes
Stadler Group, Leipzig University, Service Center: RNA Bioinformatics Center - RBC
Many small nucleolar RNAs and many of the hairpin precursors of miRNAs are processed from long non-protein-coding (lncRNA) host genes. In contrast to their highly conserved and heavily structured payload, the host genes feature poorly conserved sequences. Nevertheless, there is mounting evidence that the host genes have biological functions beyond their primary task of carrying a ncRNA as payload.
So far, no connections between the function of the host genes and the function of their payloads have been reported. Here we investigate whether there is evidence for an association of host gene function or mechanisms with the type of payload.For more information see RNA Bioinformatics Center (RBC)
Funded by: Federal Ministry of Education and Research (031A538B) and German Academic Exchange Service
Search projects by keywords: